SOTE - Chemistry Test1
0 of 20 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
Information
Hello, Dear Ones,
Trust Academy provides all registration services for the entrance exams of Hungarian universities for free.
Applicants can send a copy of their passport and email to our email address:
admission@trustacademyhu.com
Please note the following:
This test belongs to Semmelweis Medical University in Hungary.
The test consists of 20 Chemistry questions.
Each correct answer has 5 points and each wrong answer has 1 negative point.
The answer to each question is 60 seconds.
Choose the best word or phrase to fill in the blank.
” Good luck “
You have already completed the Test before. Hence you can not start it again.
Test is loading...
You must sign in or sign up to start the Test.
You have to finish following quiz, to start this Test:
Congratulations!!!" SOTE - Chemistry Test1 "
0 of 20 questions answered correctly
Your time:
Time has elapsed
Your Final Score is : 0
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
| Average score |
|
| Your score |
|
-
Chemistry Test
پاسخ های انتخابی : 0
تعداد سوالات صحیح: 0 و امتیاز شما 0
تعداد سوالات غلط : 0 و نمره منفی 0
-
Thank you for taking this test.
” Good Lock “
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- Answered
- Review
-
Question 1 of 20
1. Question
5 pointsCategory: Chemistry TestA sample of N2 gas occupies 4.48 L volume under standard conditions (1 atm, 00C).What is the mass of the sample? The atomic mass of N is 14
Correct
Incorrect
Unattempted
-
Question 2 of 20
2. Question
5 pointsCategory: Chemistry TestWhich properties are characteristic for the nonmetals?
-
- high electrical conductivity
- large ionization energy
- high electronegativity
- low electron affinity
Correct
Incorrect
Unattempted
-
-
Question 3 of 20
3. Question
5 pointsCategory: Chemistry TestConcerning 2 x 1024CO2 molecules, which statements are true? The molar mass is 44 g/mol.
-
- it is 12 moles
- it occupies 1.2 x 22.4 L volume under standard conditions
- it has a mass of 88 grams
- it consists of 3.6 x 1024 atoms
Correct
Incorrect
Unattempted
-
-
Question 4 of 20
4. Question
5 pointsCategory: Chemistry TestWhich of the following substances contain covalent bonds only?
1. BaCl2(aq)
2. CCl4(l)
3. HCl(g)
4. NH4Br(aq)Correct
Incorrect
Unattempted
-
Question 5 of 20
5. Question
5 pointsCategory: Chemistry TestWhich of the following atoms are isotopes of each other?
X: 11 protons, 11 electrons, 12 neutrons
Y: 11 protons, 10 electrons, 12 neutrons
V: 11 protons, 11 electrons, 13 neutrons
W: 12 protons, 12 electrons, 12 neutrons
Correct
Incorrect
Unattempted
-
Question 6 of 20
6. Question
5 pointsCategory: Chemistry TestAn aqueous solution is prepared by dissolving 6 g NaOH in 250 mL final volume. What is the molar concentration of the solution?
The molar mass of NaOH is 40 g/mol
Correct
Incorrect
Unattempted
-
Question 7 of 20
7. Question
5 pointsCategory: Chemistry Test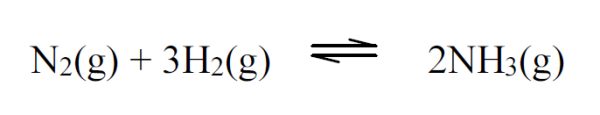
The reaction is exothermic towards product formation. Which of the following changes of conditions will shift the equilibrium of the reaction to the right?
- increase the pressure
- increase the concentration of NH3.
- increase the concentration of H2 gas
- decreasing the temperature
Correct
Incorrect
Unattempted
-
Question 8 of 20
8. Question
5 pointsCategory: Chemistry TestWhen two elements, X (atomic number 13) and Y (atomic number 8), react the compound formed will be:
Correct
Incorrect
Unattempted
-
Question 9 of 20
9. Question
5 pointsCategory: Chemistry TestWhich is the most basic solution?
Correct
Incorrect
Unattempted
-
Question 10 of 20
10. Question
5 pointsCategory: Chemistry TestWhich set contains only polar molecules?
Correct
Incorrect
Unattempted
-
Question 11 of 20
11. Question
5 pointsCategory: Chemistry TestIn any reaction where a calcium atom changes to calcium ion, the calcium atom
- has lost an electron
- has become an anion
- has been oxidized
- has achieved noble gas electron configuration
Correct
Incorrect
Unattempted
-
Question 12 of 20
12. Question
5 pointsCategory: Chemistry TestWhat is the oxidation number of Cr in K2Cr2O7
Correct
Incorrect
Unattempted
-
Question 13 of 20
13. Question
5 pointsCategory: Chemistry TestWhich group has the greatest first ionization energy?
Correct
Incorrect
Unattempted
-
Question 14 of 20
14. Question
5 pointsCategory: Chemistry TestWater solutions of the following five compounds have the same molar Arrange them in the order of increasing pH.
Correct
Incorrect
Unattempted
-
Question 15 of 20
15. Question
5 pointsCategory: Chemistry TestHow many grams of solid potassium chloride are needed to prepare 200 mL solution with 15 % m/m concentration?
The density of the solution is 1.2 g/mL.
Correct
Incorrect
Unattempted
-
Question 16 of 20
16. Question
5 pointsCategory: Chemistry TestWhich are the oxidation-reduction reactions?
1- 2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2(g)
2- 2NaOH(aq) + H2SO4(aq) -> Na2SO4(aq) + 2H2O(l)
3- Ca2+(aq) + CO3 2─(aq) -> CaCO3 (s)
4- H2(g) + Cl2(g) -> 2HCl(g)
Correct
Incorrect
Unattempted
-
Question 17 of 20
17. Question
5 pointsCategory: Chemistry TestChoose the compound with an ester group
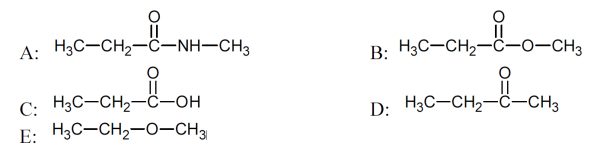 Correct
Correct
Incorrect
Unattempted
-
Question 18 of 20
18. Question
5 pointsCategory: Chemistry TestWhich pairs are structural isomers?
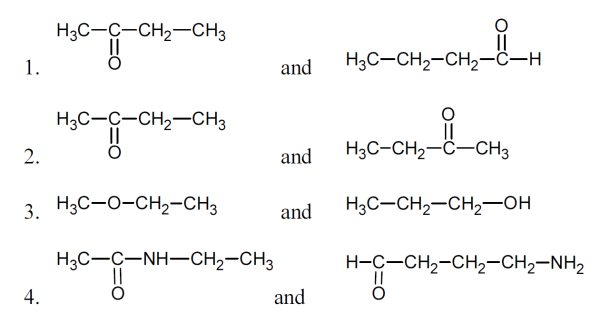 Correct
Correct
Incorrect
Unattempted
-
Question 19 of 20
19. Question
5 pointsCategory: Chemistry TestThe main product in the following reaction is:
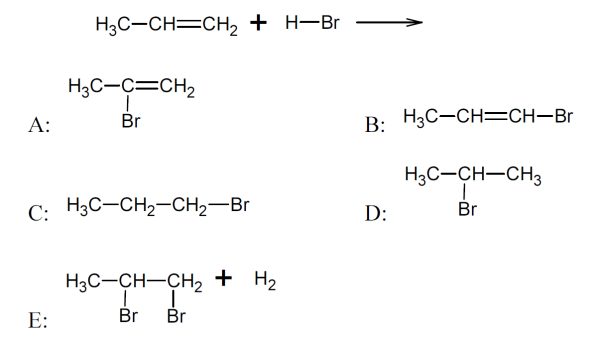 Correct
Correct
Incorrect
Unattempted
-
Question 20 of 20
20. Question
5 pointsCategory: Chemistry TestWhich statements are true for the following molecule in its open chain form?
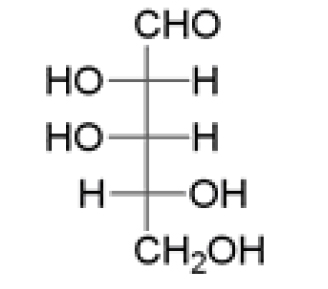
- It is an aldopentose
- It has 4 chiral carbon atoms
- It is a monosaccharide
- It is a D-sugar.
Correct
Incorrect
Unattempted